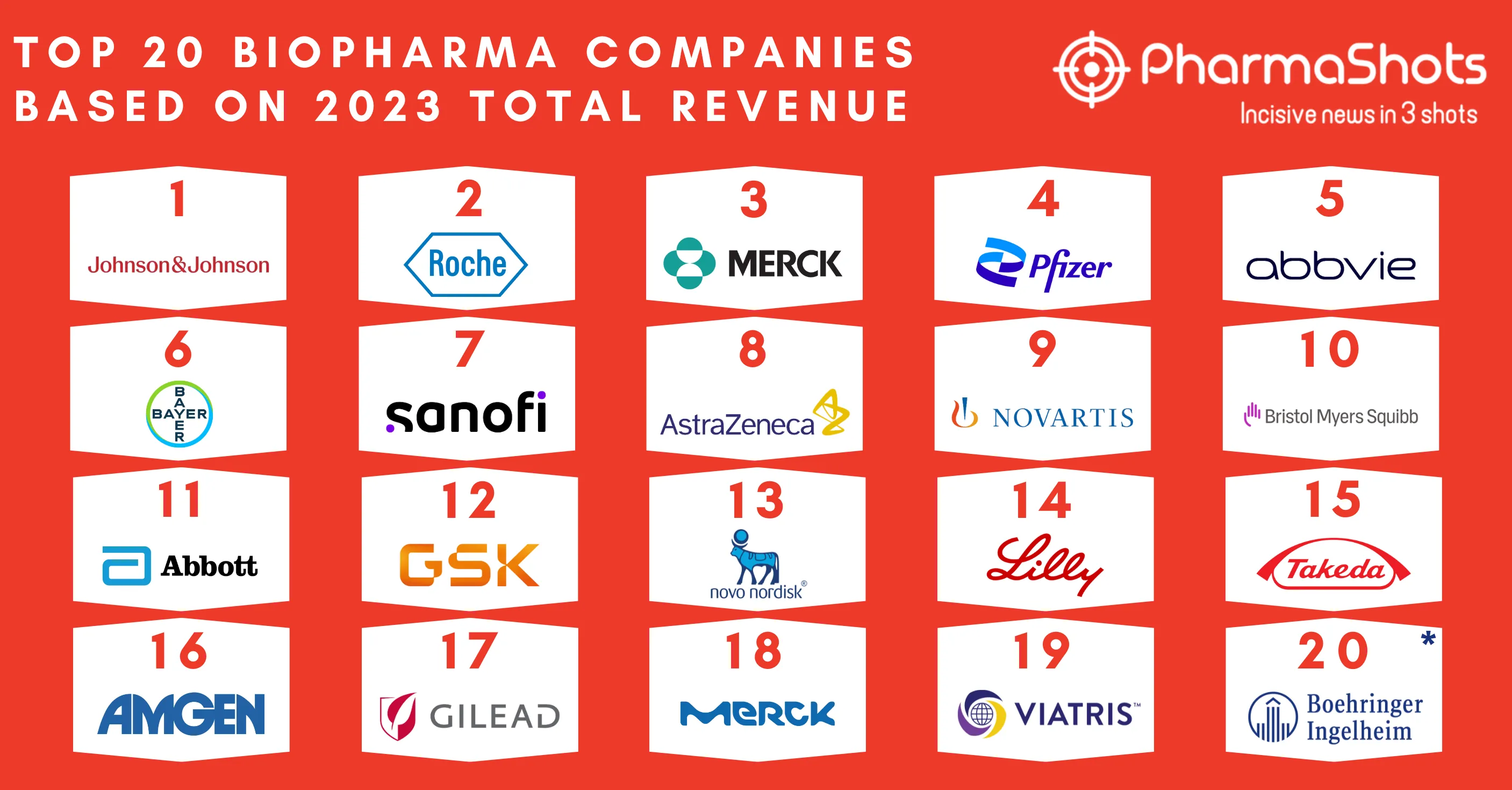
Top 20 Prescription Drugs Based on 2022 Total Revenue
Shots:
- Prescription drugs have an instrumental role in effectively controlling chronic diseases like heart-related conditions, diabetes, arthritis, hypertension, and cancer, among others. Unlike over-the-counter drugs, prescription drugs are advised not to be taken without the authorization of a doctor
- In 2022, the global market size of prescription drugs was worth $1035.5B and is envisioned to reach $1892.9B by 2030 with a CAGR of 9.0 percent. In 2022, Humira was the top-performing drug with total sales of $21.24B followed by Keytruda and Eliquis with sales of $20.94B and $18.27B respectively
- PharmaShots presents a condensed report on the Top 20 Prescription Drugs Based on 2022 Total Revenue

Proprietary Name: Eylea
Non-Proprietary Name: Aflibercept
Company: Regeneron Pharmaceuticals, Bayer
First Approved: US (Nov 18, 2011), EU (Nov 22, 2012)
Total Revenue: $3.45B
Indications Approved: Neovascular (Wet) Age-Related Macular Degeneration, Diabetic Macular Oedema, Myopic Choroidal Neovascularisation (myopic CNV), Retinopathy of Prematurity
- Approved in the form of an IV injection, Eylea is a vascular endothelial growth factor (VEGF) inhibitor indicated for the treatment of various indications, including Neovascular (Wet) Age-Related Macular Degeneration, Diabetic Macular Edema, Diabetic Retinopathy, and Retinopathy of Prematurity
- Eylea acts as a soluble decoy receptor that binds VEGF-A and PIGF thereby inhibiting the binding and activation of these cognate VEGF receptors
- The 2022 sales of Eylea rose by 3.87% as compared to 2021. The rise in sales of Eylea was attributed to the increase in volume through continued market penetration, majorly in Europe and Asia/Pacific

Proprietary Name: Veklury
Non-Proprietary Name: Remdesivir
Company: Gilead Sciences
First Approved: US (Oct 22, 2020), EU (Jul 03, 2020)
Total Revenue: $3.90B
Indications Approved: COVID-19
- Prescribed in the form of an injection, Veklury is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleotide analog RNA polymerase inhibitor indicated for the treatment of COVID-19 in adults and pediatric patients aged 28 years or above
- It is an antiviral drug that acts against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Veklury’s 2022 sales faced a recession of 29.83% as compared to 2021
- The massive fall in the product’s 2022 revenue was primarily led by a lower demand driven by reduced hospitalization rates in the U.S. and Europe

Proprietary Name: Perjeta
Non-Proprietary Name: Pertuzumab
Company: Roche
First Approved: US (Jun 08, 2012), EU (Mar 04, 2013)
Total Revenue: $4.42B
Indications Approved: HER2-positive metastatic breast cancer (MBC), Inflammatory
- Perjeta is a humanized monoclonal antibody approved in the form of an IV injection to treat HER2-Positive Metastatic Breast Cancer and Inflammatory Disorders
- It targets the extracellular dimerization domain (Subdomain II) of the HER2 protein and thereby blocks ligand-dependent heterodimerization of HER2 and other HER family members, including EGFR, HER2, and HER4.
- In 2022, Perjeta’s total sales increased by 1.93% as compared to 2021. The sales of Perjeta rose in 2022 primarily due to its higher demand in China for the treatment of early and metastatic breast cancer and was also led by the increased sales of the product across the US

Proprietary Name: Ibrance
Non-Proprietary Name: Palbociclib
Company: Pfizer
First Approved: US (Feb 03, 2015), EU (Nov 09, 2016)
Total Revenue: $5.12B
Indications Approved: Breast Cancer
- An oral kinase inhibitor, Ibrance, is indicated in the form of an oral tablet to treat patients with hormone receptor (HR)-positive, Human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer
- Ibrance is prescribed in combination with an aromatase inhibitor as initial endocrine-based therapy and with fulvestrant in patients with disease progression following endocrine therapy. It inhibits cyclin-dependent kinases (CDK) 4 and 6. Its total sales in 2022 dropped by 5.83% as compared to 2021
- The sales of Ibrance dropped in 2021 primarily due to prior-year clinical trial purchases internationally, planned price decreases, and continued increase in the proportion of patients accessing Ibrance through the U.S. Patient Assistance Program

Proprietary Name: Enbrel
Non-Proprietary Name: Etanercept
Company: Amgen, Pfizer, Takeda
First Approved: US (Nov 02, 1998), EU (Feb 03, 2000)
Total Revenue: $5.12B
Indications Approved: Rheumatoid Arthritis, Polyarticular Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Plaque Psoriasis
- A subcutaneous dimeric fusion protein, Enbrel contains an extracellular ligand-binding portion of the human 75-kilodalton (p75) TNFR linked to the Fc portion of human IgG1. It is approved to treat adult patients with moderate to severely active rheumatoid arthritis
- Enbrel prevents TNF- and TNF- (lymphotoxin alpha [LT-]) from binding to cell surface TNFRs, leaving TNF inactive. Enbrel is jointly commercialized by Amgen, Pfizer, and Takeda
- In 2022, Enbrel’s total sales declined by 9.38% as compared to 2021. The dip in the product’s sales was attributed to the unfavorable changes to estimated sales deduction, lower volume, and lower net selling price

Proprietary Name: Tagrisso
Non-Proprietary Name: Osimertinib
Company: AstraZeneca
First Approved: US (Nov 13, 2015), EU (Apr 24, 2017)
Total Revenue: $5.44B
Indications Approved: Non-Small Cell Lung Cancer
- Tagrisso is an orally indicated kinase inhibitor prescribed to treat adult patients with Non-Small Cell Lung Cancer whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations
- It inhibits the epidermal growth factor receptor (EGFR) that binds irreversibly to the mutant forms of EGFR. Moreover, it is approved as an adjuvant therapy post-tumor resection to treat Non-Small Cell Lung Cancer
- Tagrisso’s 2022 total revenue spiked by 8.55% vs 2021. The rise in sales in 2022 was attributed to the increased use of Tagrisso across all markets

Proprietary Name: Ocrevus
Non-Proprietary Name: Ocrelizumab
Company: Roche
First Approved: US (Mar 28, 2017), EU (Jan 08, 2018)
Total Revenue: $6.52B
Indications Approved: Multiple Sclerosis
- Ocrevus is a humanized monoclonal antibody, approved in the form of an IV injection to treat both relapsing and primary progressive forms of multiple sclerosis. It is a monoclonal antibody that targets CD20-positive B cells, an immune cell type that has a significant contribution to myelin and axonal damage
- Ocrevus triggers antibody-dependent cellular cytolysis and complement-mediated lysis
- In 2022, the total sales of Ocrevus went up by 17.78% as compared to 2021. The increased sales of Ocrevus in 2022 were led by the increase in sales by new and returning patients with higher proportions of sales contributed by the returning patients

Proprietary Name: Xarelto
Non-Proprietary Name: Rivaroxaban
Company: Johnson & Johnson, Bayer
First Approved: US (Jul 01, 2011), EU (Sep 30, 2008)
Total Revenue: $7.32B
Indications Approved: Deep Vein Thrombosis Prophylaxis after Knee Replacement Surgery, Deep Vein Thrombosis Prophylaxis after Hip Replacement Surgery, Prevention of Thromboembolism in Atrial Fibrillation, Coronary Artery Disease, Peripheral Arterial Disease, Deep Vein Thrombosis, Pulmonary Embolism, Venous Thromboembolism
- A factor Xa inhibitor, Xarelto, is approved as a tablet for the treatment of multiple indications, including stroke and systemic embolism in nonvalvular atrial fibrillation, deep vein thrombosis, pulmonary embolism, etc. Xarelto functions as a selective inhibitor of FXa and does not require a cofactor for its activity
- Though it has no direct effect on platelet aggregation, it indirectly inhibits platelet aggregation induced by thrombin. Xarelto is commercialized by both J&J (North America) and Bayer (Rest of the World)
- In 2022, the sales of Xarelto decreased by 6.45% as compared to 2021. The decline in sales in 2022 was attributed to the tender procedures in China, price pressure in the United Kingdom, and the expiration of its patent in Brazil

Proprietary Name: Trulicity
Non-Proprietary Name: Dulaglutide
Company: Eli Lilly
First Approved: US (Sep 18, 2014), EU (Nov 21, 2014)
Total Revenue: $7.44B
Indications Approved: Type 2 Diabetes, Cardiovascular Risk Reduction
- Approved in the form of an injection for subcutaneous use, Trulicity is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated for the treatment of Type 2 Diabetes and Cardiovascular Risk Reduction
- It is recommended to be taken with a suitable diet and exercise for diabetes control. Trulicity, dulaglutide, is a human GLP-1 receptor agonist that activates the GLP-1 receptor, a membrane-bound cell-surface receptor coupled to adenylyl cyclase in pancreatic beta cells, and increased intracellular cyclic AMP (cAMP) in beta cells leading to glucose-dependent insulin release
- Trulicity’s total revenue in 2022 increased by 14.95% as compared to 2021. An increased demand drove the rise in the product's total revenue, which was partially balanced, by lower realized prices as a result of an unfavorable segment mix and greater contracted rebates

Proprietary Name: Trikafta/Kaftrio
Non-Proprietary Name: Combination of Ivacaftor, Tezacaftor, and Elexacaftor
Company: Vertex
First Approved: US (Oct 21, 2019), EU (Aug 21, 2020)
Total Revenue: $7.69B
Indications Approved: Cystic Fibrosis
- Trikafta is an oral drug developed through a combination of ivacaftor (a CFTR potentiator), tezacaftor, and elexacaftor, approved for the treatment of cystic fibrosis in patients aged 2 years and older
- This combination therapy is approved in the US by the name Trikafta and in the EU by Kaftrio. Trikafta binds to different sites on the CFTR protein
- In 2022, the sales of Trikafta, boosted by 34.92% as compared to 2021. The significant surge in product sales was primarily driven by the strong uptake of Trikafta/Kaftrio in global markets and a consistent performance of Trikafta in the United States, following the June 2021 launch of Trikafta for children with CF 6 through 11 years of age

Proprietary Name: Darzalex
Non-Proprietary Name: Daratumumab
Company: Johnson & Johnson
First Approved: US (Nov 16, 2015), EU (Apr 28, 2017)
Total Revenue: $7.98B
Indications Approved: Multiple Myeloma
- A human anti-CD38 monoclonal antibody, Darzalex is developed and marketed by Janssen Pharmaceuticals as a combination of a CD38-directed cytolytic antibody, daratumumab, and an endoglycosidase, hyaluronidase
- It is prescribed in the form of an IV infusion to treat Multiple Myeloma. Daratumumab functions by binding to CD38, leading to the cell's apoptosis through antibody-dependent cellular cytotoxicity, complement-dependent toxicity, inhibition of mitochondrial transfer, or antibody-dependent cellular phagocytosis
- In 2022, Darzalex’s revenue boosted by 32.44% vs 2021. The rise in the product’s total sales in 2022 was led by continued strong market growth and share gains in all regions

Proprietary Name: Opdivo
Non-Proprietary Name: Nivolumab
Company: Bristol-Myers Squibb
First Approved: US (Dec 22, 2014), EU (Jun 19, 2015)
Total Revenue: $8.25B
Indications Approved: Melanoma, Non-Small Cell Lung Cancer, Malignant Pleural Mesothelioma, Renal Cell Carcinoma, Classical Hodgkin Lymphoma, Squamous Cell Carcinoma of the Head and Neck, Urothelial Carcinoma, Colorectal Cancer, Hepatocellular Carcinoma, Esophageal Cancer, Gastric Cancer, Gastroesophageal Junction Cancer, and Esophageal Adenocarcinoma.
- Opdivo is a human immunoglobulin G4 (IgG4) monoclonal antibody prescribed in the form of an intravenous infusion for the treatment of Melanoma, Non-Small Cell Lung Cancer, Malignant Pleural Mesothelioma, Renal Cell Carcinoma, Classical Hodgkin Lymphoma, Head and Neck Cancer, Urothelial Carcinoma, etc.
- Opdivo binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, resulting in decreased tumor growth
- Opdivo’s total sales in 2022 increased by 9.65% as compared to 2021. The rise in the sales of Opdivo was led by its higher demand in the US across multiple indications incl. NSCLC

Proprietary Name: Imbruvica
Non-Proprietary Name: Ibrutinib
Company: Johnson & Johnson, AbbVie
First Approved: US (Nov 13, 2013), EU (Oct 21, 2013)
Total Revenue: $8.35B
Indications Approved: Chronic Lymphocytic Leukemia, Waldenström Macroglobulinemia, Graft-versus-host disease, Lymphoma
- A kinase inhibitor, Imbruvica, is approved for the treatment of various indications, including Chronic Lymphocytic Leukemia, Waldenström Macroglobulinemia, Graft-versus-host disease, and Lymphoma
- It is a small molecule inhibitor of Bruton’s tyrosine kinase (BTK) as it forms a covalent bond with a cysteine residue in the BTK active site, thereby leading to the inhibition of BTK enzymatic activity. Imbruvica is co-developed by Cilag (J&J) and Pharmacyclics (AbbVie). Moreover, J&J is responsible for the sales of Imbruvica across the globe and AbbVie is responsible for the sales of Imbruvica in the US
- Imbruvica’s 2022 revenue declined by 14.57% as compared to 2021. This fall in the product’s sales could be attributed to the decreased market demand and lower market share in the US and partially due to increased collaboration revenues

Proprietary Name: Dupixent
Non-Proprietary Name: Dupilumab
Company: Regeneron Pharmaceuticals
First Approved: US (Mar 28, 2017), EU (Sep 27, 2017)
Total Revenue: $8.9B
Indications Approved: Atopic Dermatitis, Asthma, Chronic Rhinosinusitis with Nasal Polyposis, Eosinophilic Esophagitis, Prurigo Nodularis
- Dupixent is an interleukin-4 receptor alpha antagonist, administered as a subcutaneous injection. It is a monoclonal antibody that is available as a single-dose pre-filled pen or a single-dose pre-filled syringe
- Dupixent is used for the treatment of multiple indications, including Atopic Dermatitis, Asthma, Eosinophilic Esophagitis, etc. Its mechanism involves inhibiting the signaling of the IL-4 and IL-13 pathways
- Dupixent’s 2022 revenue recorded a strong increase of 49.05% as compared to 2021. As Dupixent prescriptions for various indications continued to expand across the globe in 2022, the product's sales totals grew

Proprietary Name: Stelara
Non-Proprietary Name: Ustekinumab
Company: Janssen Pharmaceutical
First Approved: US (Sep 25, 2009), EU (Jan 15, 2009)
Total Revenue: $9.72B
Indications Approved: Plaque Psoriasis, Crohn’s Disease, Psoriatic Arthritis (PsA), Ulcerative colitis
- Approved in the form of an SC or IV injection, Stelara is indicated for the treatment of various indications, including Plaque Psoriasis, Crohn’s Disease, Psoriatic Arthritis (PsA), and Ulcerative colitis
- It is an interleukin inhibitor that blocks certain proteins in the body called IL-12 (interleukin-12) and IL-23 (interleukin-23) responsible for inflammation, swelling, and skin symptoms in these indications
- Compared to 2021, Stelara’s total sales rose by 6.45% in 2022. This growth can be attributed to a strong acceptance of Stelara in Crohn’s disease and Ulcerative Colitis

Proprietary Name: Revlimid
Non-Proprietary Name: Lenalidomide
Company: BMS, BeiGene
First Approved: US (Dec 27, 2005), EU (Jun 14, 2007)
Total Revenue: $10.06B
Indications Approved: Myelodysplastic Syndrome, Multiple Myeloma, Lymphoma, Follicular Lymphoma
- Revlimid is an orally approved thalidomide analogue prescribed for the treatment of patients with certain cancers and serious conditions affecting blood cells and bone marrow, including Multiple Myeloma, Myelodysplastic Syndromes, and Mantle Cell Lymphoma
- Lenalidomide functions as an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. The sales of Revlimid are managed by BMS globally except in Asia for which BieGene is held responsible
- In 2022, the total sales of Revlimid went down by 21.98% as compared to 2021. The fall in the sales of Revlimid in 2022 led primarily to lower demand for the products as a result of generic erosion, foreign exchange, and lower average net selling prices

Proprietary Name: Biktarvy
Non-Proprietary Name: Combination of Bictegravir, Emtricitabine, and Tenofovir Alafenamide
Company: Gilead Sciences
First Approved: US (Jun 21, 2018), EU (Feb 07, 2018)
Total Revenue: $10.39B
Indications Approved: HIV Infection
- Prescribed in an oral form, Biktarvy is an antiviral agent developed through a combination of bictegravir (an HIV-1 integrase strand transfer inhibitor), emtricitabine (FTC), and tenofovir alafenamide (TAF)
- It is indicated for the treatment of HIV infection. Both emtricitabine and tenofovir alafenamide are HIV-1 nucleoside analogue reverse transcriptase inhibitors
- In 2022, Biktarvy’s total sales boosted by 20.48% as compared to 2021. The rise in the product’s sales was attributed to the continued higher demand for Biktarvy worldwide, favorable pricing dynamics, and royalties

Proprietary Name: Eliquis
Non-Proprietary Name: Apixaban
Company: BMS, Pfizer
First Approved: US (Dec 28, 2012), EU (May 18, 2011)
Total Revenue: $18.27B
Indications Approved: Prevention of Thromboembolism in Atrial Fibrillation, Deep Vein Thrombosis Prophylaxis after Knee Replacement Surgery, Deep Vein Thrombosis Prophylaxis after Hip Replacement Surgery
- Eliquis is a factor Xa inhibitor prescribed in an oral form to lower the risk of stroke or a blood clot in patients with atrial fibrillation. Both BMS (Rest of the World) and Pfizer (Certain Small Countries) are responsible for the commercialization of Eliquis whereas Pfizer funds between 50% and 60% of all development costs depending on the study
- The sales of Eliquis in 2022 increased by 9.18% as compared to 2021
- The increased sales of Eliquis in 2022 were led by continued oral anti-coagulant adoption and market share gains in non-valvular atrial fibrillation in the US and certain markets in Europe, as well as favorable changes in channel mix in the US

Proprietary Name: Keytruda
Non-Proprietary Name: Pembrolizumab
Company: Merck & Co.
First Approved: US (Sep 04, 2014), EU (Jul 15, 2015)
Total Revenue: $20.94B
Indications Approved: Melanoma, Non-Small Cell Lung Cancer, Head and Neck Squamous Cell Cancer, Classical Hodgkin Lymphoma, Primary Mediastinal Large B-Cell Lymphoma, Urothelial Carcinoma, Gastric Cancer, Esophageal Cancer, Cervical Cancer, Hepatocellular Carcinoma, Merkel Cell Carcinoma, Renal Cell Carcinoma, Endometrial Carcinoma, etc.
- Keytruda is a monoclonal antibody injection, which blocks the PD-1 (programmed death receptor-1) receptor and helps the immune system slow or stop the growth and spread of cancer cells in the body. It functions by binding to the PD-1 receptor found on T cells and thereby inhibiting T cell proliferation and cytokine production
- A type of immunotherapy, Keytruda is approved to treat patients with Melanoma, Non-Small Cell Lung Cancer, Head, and Neck Squamous Cell Cancer Classical Hodgkin Lymphoma, etc.
- Keytruda’s net sales increased by 21.82% in 2022 compared to 2021. The sales of Keytruda in 2022 grew primarily due to a higher demand for the product as Merck continued to launch Keytruda with multiple new indications globally

Proprietary Name: Humira
Non-Proprietary Name: Adalimumab
Company: AbbVie
First Approved: US (Dec 31, 2002), EU (Sep 8, 2003)
Total Revenue: $21.24B
Indications Approved: Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Crohn’s Disease, Ulcerative Colitis, Plaque Psoriasis, Hidradenitis Suppurativa, Uveitis
- Humira is a recombinant human IgGl monoclonal antibody that prevents inflammation by specifically targeting and inhibiting TNF-α. It is approved in several indications including Rheumatoid arthritis, Psoriatic arthritis, Ulcerative Colitis, and Plaque psoriasis.
- Humira functions by binding to TNF-alpha and thereby blocks its interaction with the p55 and p75 cell surface TNF receptors. Humira’s revenue increased by 2.62% in 2022 over 2021
- Many of Humira's biosimilars are expected to hit the market this year, as the Humira patent just expired in 2023. The sales of Humira in the year 2022 rose due to a favourable market growth across therapeutic categories
Sources:
- Annual reports
- SEC Filings
- Press releases
- Product websites
Currency Conversion: X-Rates (20th Nov, 2022)
Note:
- All revenues are reported in $B
- The total revenues of Eliquis and Imbruvica include collaborative revenues
Related Posts: Top 20 Prescription Drugs Based on 2021 Total Revenue
Tags

Shivani is a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.